Sulfur Nutrition for Mississippi Crops and Soils
Sulfur (S) is an essential plant nutrient. Among the amino acids that make up proteins, sulfur is a component of cysteine and methionine. Agronomic crops produced in Mississippi remove six to 32 pounds of sulfur per acre. Table 1 outlines sulfur removal by agronomic crops across the state of Mississippi.
Table 1. Sulfur removal by Mississippi agronomic crops.
|
Crop |
Yield/units |
Sulfur removal (lb/acre) |
|
Bermudagrass |
8 tons/acre |
32 |
|
Corn |
200 bushels/acre |
16 |
|
Cotton (seed & lint) |
2600 lbs/acre |
5 |
|
Cotton (other parts) |
3000 lbs/acre |
15 |
|
Fescue |
3.5 tons/acre |
20 |
|
Peanuts (nuts) |
4000 lbs/acre |
10 |
|
Peanuts (vines) |
5000 lbs/acre |
11 |
|
Soybeans |
60 bushels/acre |
11 |
|
Wheat |
60 bushels/acre |
6 |
Adopted from Gatiboni and Hardy 2019, The Fertilizer Institute 2020.
Sulfur present in soils is chiefly in organic forms. Whether the sulfur is present, or added by manures, the organic S must be mineralized, or converted by microbes to the sulfate ion (SO42-). Sulfate form S is practically the only S used by plants and is taken in through the roots of the plant. The rate of mineralization depends on temperature and moisture in the soil; the rate will increase as temperatures increase during the summer growing season.
For many years, atmospheric deposition provided sufficient sulfur to sustain crop yields. Less coal use, improved smokestack scrubbing technology, and widespread use of automobile catalytic converters has decreased S emissions. Correspondingly, sulfur deposition on soils from the atmosphere also decreased significantly (Figure 1, multiply by 0.8922 to convert to pounds per acre). Another source of plant-available sulfur was pesticides and certain phosphorus fertilizers; however, they are used less or no longer applied to agricultural land altogether.
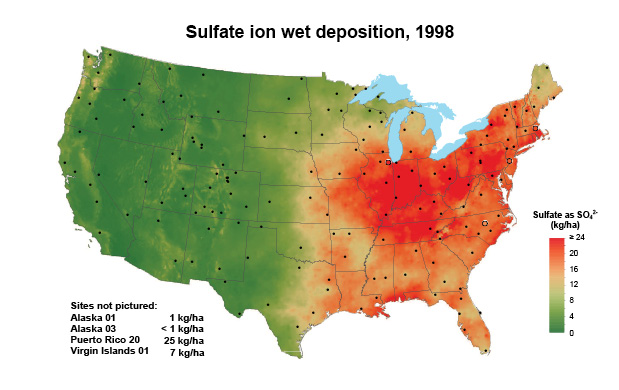
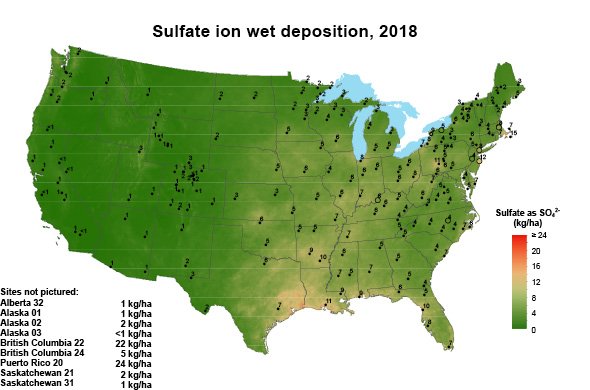
Figure 1. Sulfur deposition as sulfate in 1998 and 2018. Adopted From the National Atmospheric Deposition Program.
Plant-available sulfate ion is prone to leaching, movement downward through the profile with water, similarly to nitrate form nitrogen loss. Accordingly, leaching loss is more likely in coarse textured, sandy soils.
Soil Testing
Soil testing for sulfur is not widely used in our warm, humid southern climate. Testing soils for S is hampered by traits shared with nitrate-form nitrogen such as mineralization from organic matter and vulnerability to leaching. Soil tests for phosphorus and potassium are widely available that categorize plant-available nutrient levels in soils as a basis for relevant recommendations that should provide crop responses. Unlike phosphorus and potassium, sulfur response data for similar calibration and correlation for S has lacked predictability.
Even with the challenges, public soil testing laboratories in Tennessee, South Carolina, and North Carolina provide some sulfur soil testing services. The Mississippi State University Extension Soil Testing Laboratory estimates soil S if organic matter is requested; this estimate is based on organic matter originated from studies completed in Mississippi.
Sulfur Deficiency
Pale green to yellow young leaves signal sulfur deficiency. Older leaves on the same plant may be darker as S does not relocate within growing plants. S-deficient plants will be spindly and small with slow growth and delayed fruiting (Figure 2).


Figure 2. Normal soybean plant and sulfur deficient soybean plant. Photos by Carl R. Crozier, PhD, North Carolina State University Professor Emeritus and Extension Specialist, Department of Crop and Soil Sciences.
Sulfur deficiencies often occur on sandy, low cation exchange capacity soils, and/or low organic matter soils on ridges. Early season deficiencies may not lead to lower yields because warming conditions through the season will stimulate microbial transition of organic S to the plant-available sulfate form.
Plant tissue analysis, particularly the ratio of nitrogen and sulfur concentrations in the plants, may confirm or deny S deficiency. Producers should sample both ‘good’ and ‘bad’ spots in the field for a comparison. Confirmed deficiencies can be addressed early in the growing season with a fertilizer containing readily available S such as sulfate. However, note the field for future attention to sulfur management prior to the subsequent crop.
Sulfur fertilization
Several sources of fertilizer sulfur are available; see Table 2 for a comprehensive list. With the lack of soil test correlation and calibration information, application rates should be based on the crop grown and soil properties including texture and organic matter content, as well as site-specific field factors such as slope.
Table 2. Fertilizers and soil amendments that contain sulfur.
|
Material |
N-P-K-S Analysis |
|
Elemental Sulfur |
0-0-0-90S |
|
Ammonium Thiosulfate |
12-0-0-26S |
|
Ammonium Sulfate |
21-0-0-24S |
|
Calcium Sulfate (Gypsum) |
0-0-0-17S |
|
N-P-S products |
13-33-0-15S |
|
12-40-0-10S-1Zn |
|
|
12-33-0-15S |
|
|
10-46-0-9S |
|
|
9-43-0-16S |
|
|
Polysulfate |
0-0-14-19S |
|
Magnesium Sulfate |
0-0-0-14S |
|
Potassium Magnesium Sulfate |
0-0-22-23S |
|
Potassium Sulfate |
0-0-50-18S |
|
Mono Ammonium Phosphate |
11-52-0-1.5S |
|
Diammonium Phosphate |
18-46-0-1.4S |
By-product sources of sulfur, such as animal manures, lysine manufacturing, soybean soapstock processing, wallboard (gypsum), and flue-gas desulfurization all have varying levels N-P-K values.
Readily available (in the plant nutrition sense of available) S sources include ammonium sulfate, potassium sulfate, gypsum, and zinc sulfate. A slower-acting sulfur source is elemental S fertilizer, which must be oxidized through microbial processes to plant-available SO42-. This process takes several months. Management of ammonium sulfate and elemental sulfur should consider that these sources acidify soil when applied. Several nitrogen-phosphorus-sulfur products have entered the market and are being evaluated by Extension across the country. Work with vendors to determine the right sulfur fertilizer for the situation while considering solubility, soil acidification, and other fertilizer properties.
Summary
Factors that affect sulfur crop nutrition are:
- Soil organic matter levels.
- Land application of sulfur containing soil amendments, including manures.
- Less atmospheric deposition.
- Leaching potential in coarse textured, sandy soils.
- Crop removal rates through yield increases.
- Lack of calibrated and correlated soil sulfur tests.
In the growing season, farmers and consultants should note visual sulfur deficiency symptoms, use tissue testing to confirm suspicions, and consider economical remedial in-season actions. The lack of a consistent soil test for S for warm humid conditions presents challenges, however producer crop plans, soil and field information, and Tables 1 and 2 can help determine the right amount of the right fertilizer to use at the right place and the right time.
More information about soil nutrient management is available from county MSU Extension offices and online at Inorganic Fertilizers for Crop Production and Nutrient Management Guidelines for Agronomic Crops Grown in Mississippi.
References
Gatiboni, L. & D. H. Hardy. (2019). Sulfur Fertilization of North Carolina Crops. North Carolina State University Cooperative Extension Crop and Soil Sciences. Accessed online. https://content.ces.ncsu.edu/sulfur-fertilization-of-north-carolina-crops
The Fertilizer Institute. (2020). Sulfur. Essential Elements. Accessed online. https://www.tfi.org/our-industry/intro-to-fertilizer/nutrient-science.
Publication 3669 (POD-07-21)
By Larry Oldham, PhD, Extension Professor, Plant and Soil Sciences.
The Mississippi State University Extension Service is working to ensure all web content is accessible to all users. If you need assistance accessing any of our content, please email the webteam or call 662-325-2262.

